BY RESEARCHERS, FOR RESEARCHERS
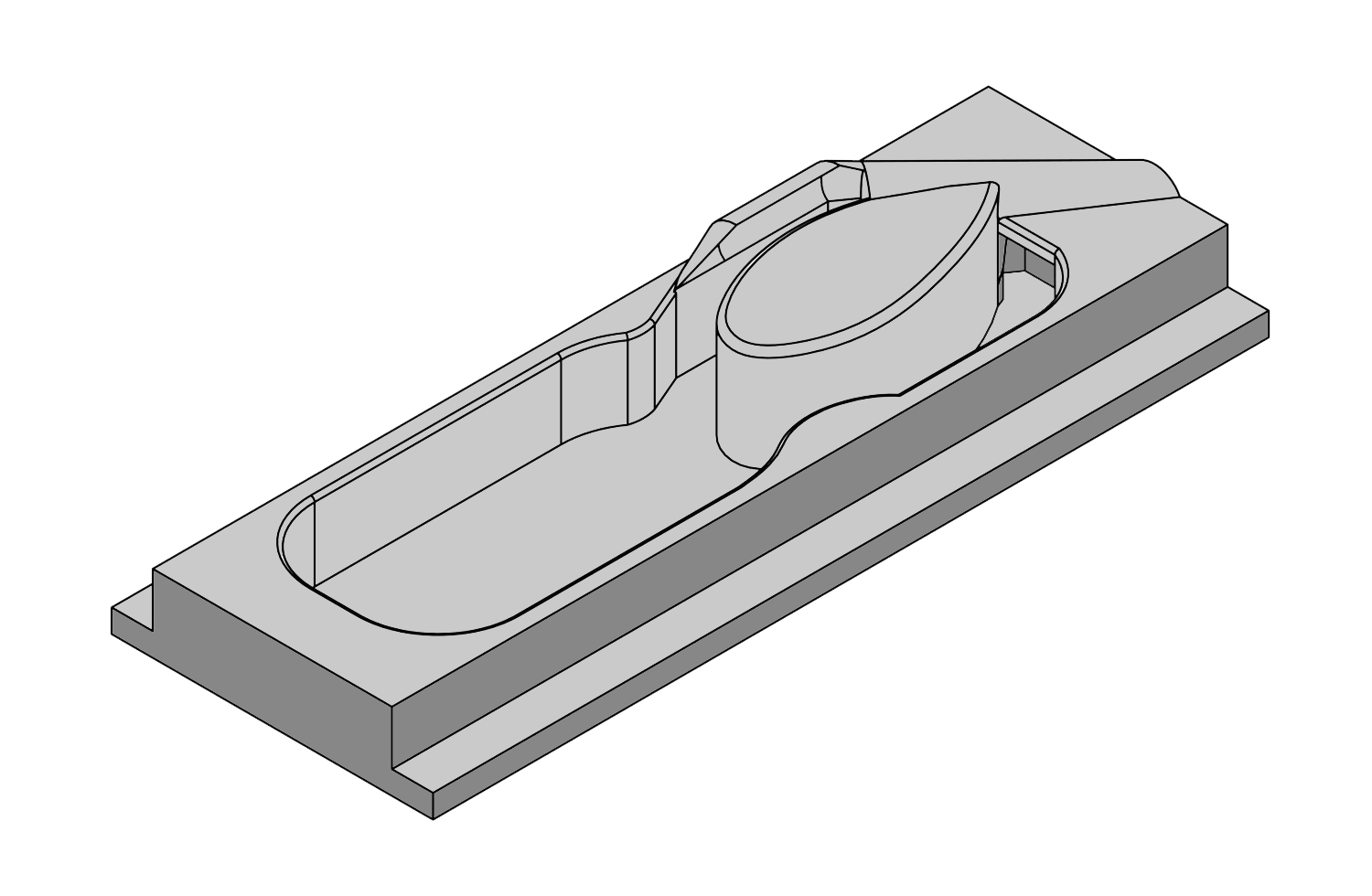
L-VIS (ULTRA-LOW VOLUME IN-VITRO SLICE CHAMBER)
PopNeuron’s L-VIS (Ultra-Low Volume In-Vitro Slice Chamber) marks a significant advancement in neuroscience research. This innovative chamber requires only 1.5 to 2 ml of artificial cerebro-spinal fluid (ACSF) for experiments, drastically reducing the volume compared to other designs. By eliminating external containers and tubes, it facilitates continuous reoxygenation and recirculation of ACSF directly in the chamber, ensuring efficient oxygen supply and cost savings, especially when using expensive pharmacological additives. Rigorously tested through fluid dynamics modeling, real-life experiments with dyes and beads, and comprehensive tissue health assessments, this chamber offers a practical, cost-effective solution for intricate neuroscience studies, maximizing the potential of live tissue analysis.
What Makes L-VIS Different
Ultra-Low ACSF Volume Requirement: The chamber uses only 1.5 to 2 ml of artificial cerebro-spinal fluid (ACSF), significantly reducing the quantity needed for experiments.
Innovative Design: Eliminates external containers and tubes, integrating a system for direct reoxygenation and recirculation of ACSF within the chamber.
Cost-Effective Solution: Particularly beneficial when using expensive pharmacological substances, as it minimizes the amount of ACSF and additives required.
Rigorous Testing and Validation: Fluid dynamics modeled and validated through real-life testing with dyes and beads, ensuring efficient operation and solution movement.
Proven Tissue Health Maintenance: Extensively tested for oxygen saturation and solution stability, confirming the chamber’s effectiveness in maintaining tissue health over extended periods.

BY RESEARCHERS, FOR RESEARCHERS
See L-VIS Rendered in 3D Model
L-VIS (Ultra-Low Volume In-Vitro Slice Chamber)
Many experimental approaches in medicine or biology, and particularly in neuroscience, require the use of live tissue. Ex-vivo tissue samples, such as brain slices, are prepared and then kept alive in artificial cerebro-spinal fluid (ACSF), a solution containing the most important ingredients of actual cerebro-spinal fluid. Additionally, it has sugar added as an energy source and is bubbled with oxygen to keep the tissue alive. While the tissue is under a microscope for examination, for example, through patch clamp recordings, it is continuously bathed in ACSF, which is rapidly exchanged to ensure there is always enough oxygen available for the tissue. From a practical point of view, the chamber in which the tissue is located (the recording chamber) has an inflow and an outflow connected with tubes to holding containers for continuous fluid exchange.
In its simplest form, ACSF contains just a few inexpensive ingredients, and even liters of it cost very little to make. Therefore, discarding the solution after it has been used in the recording chamber is not a problem. However, whenever pharmacological ingredients such as ion channel blockers, receptor blockers, or similar reagents are added to the solution, expenses can increase significantly – to the point where just one day of recording can cost hundreds or even thousands of dollars. In these cases, investigators try to reduce the amount of ACSF as much as possible – for example, by designing smaller containers or reducing flow rates. Additionally, they recycle the solution rather than discarding it after a single pass through the recording chamber.
PopNeuron’s L-VIS takes this concept to the limit of what is possible. Our chamber uses about 1.5 to 2 ml of ACSF per experiment, which is several-fold less than any other design on the market or what can be custom-designed by investigators. The main reason for the small volume is that we eliminated external containers and tubes with the solution altogether, taking a literal approach to “out of the box” thinking. Our chamber requires a single fill with ACSF. The solution is then continuously reoxygenated through a small tube that carries just the gas into the chamber. A water pump design recirculates ACSF within the chamber, mixes and reoxygenates the solution, and moves it across the tissue in a figure-8 movement pattern.
How do we know this design works?
The fluid dynamics were modeled, and the chamber design was adjusted in multiple iterative steps. Once a design looked feasible, a prototype was produced and tested in real life with dyes and small beads transported in the fluid. Videos of these motions were carefully analyzed, and the results were used to further optimize the design, followed by more modeling and more real-life testing. Oxygen saturation of the solution was tested over multiple hours to measure the ability of the water pump mechanisms to oxygenate the solution. Liquid chromatography and mass spectroscopy were used to test for potential changes in the solution over time and control for possible accumulation of waste products. Finally, patch clamp recordings were performed over several hours to test for the overall health of the tissue.
Learn More About In-Vitro Slice Chambers and L-VIS with our Mini-Series:
Documentation and Software
By using PopNeuron software, you agree to our End-User License Agreement, available here.
Publications:
Dondzillo A, Quinn KD, Cruickshank-Quinn CI, Reisdorph N, Lei TC, Klug A. A recording chamber for small volume slice electrophysiology. J Neurophysiol. 2015 Sep;114(3):2053-64. doi:10.1152/jn.00289.2014. Epub 2015 Jul 22. PMID: 26203105; PMCID: PMC4588903.
Patent:
Dondzillo, A. Klug, and T. Lei: System and Methods for Conducting In-Vitro Experiments. Awarded 10-4-2016. U.S. Patent Number 9,458,420.
Terms and Conditions
Please read these Terms and Conditions (“Terms”, “Terms and Conditions”) carefully before using the website and purchasing any products from PopNeuron LLC (“us”, “we”, or “our”).
- Agreement to Terms
By accessing or using our website and purchasing any products from us, you agree to be bound by these Terms. If you disagree with any part of the terms, then you may not access the website or initiate a purchase.
- Products
- Our products are intended for use in accordance with applicable regulations and guidelines. It is your responsibility to ensure that the products purchased are suitable for your intended use.
- We strive to accurately represent our products, including descriptions, images, and specifications, but we do not guarantee that such information is complete, current, or error-free.
- Orders and Payment
- By placing an order through our website, you warrant that you are legally capable of entering into binding contracts and that all information you provide is accurate and complete.
- Prices for our products are subject to change without notice. We reserve the right to modify or discontinue any product at any time without prior notice.
- Payment must be made in full at the time of purchase. We accept various forms of payment as indicated on our website.
- Shipping and Delivery
- We aim to ship orders promptly; however, delivery times may vary depending on factors beyond our control. We are not liable for any delays in delivery.
- Risk of loss and title for products purchased from us pass to you upon delivery of the items to the carrier.
- Returns and Refunds
- Please refer to our Warranty and Return Policy [link] for information on returns and refunds.
- Warranty
- Our products may be subject to warranties provided by the manufacturer. Please refer to the specific product documentation for warranty details, as well as our Warranty and Return Policy [link].
- Limitation of Liability
- In no event shall PopNeuron LLC, nor its directors, employees, partners, agents, suppliers, or affiliates, be liable for any indirect, incidental, special, consequential, or punitive damages, including without limitation, loss of profits, data, use, goodwill, or other intangible losses, resulting from (i) your access to or use of or inability to access or use the website; (ii) any conduct or content of any third party on the website; (iii) any content obtained from the website; and (iv) unauthorized access, use, or alteration of your transmissions or content, whether based on warranty, contract, tort (including negligence), or any other legal theory, whether or not we have been informed of the possibility of such damage, and even if a remedy set forth herein is found to have failed of its essential purpose.
- Governing Law
- These Terms shall be governed and construed in accordance with the laws of Colorado, without regard to its conflict of law provisions.
- Changes to Terms
- We reserve the right, at our sole discretion, to modify or replace these Terms at any time. If a revision is material, we will try to provide at least 30 days’ notice prior to any new terms taking effect. What constitutes a material change will be determined at our sole discretion.
- Contact Us
- If you have any questions about these Terms, please contact us at frontdesk@popneuron.com.
By using our website and purchasing our products, you acknowledge that you have read, understood, and agree to be bound by these Terms and Conditions.